|
 |
|
FT-IR Spectroscopy
FT-IR Spectroscopy
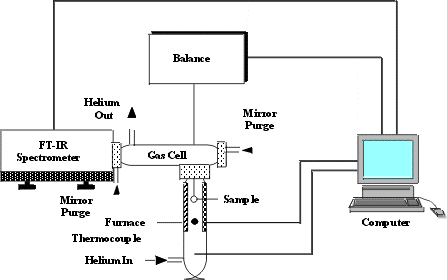
Infrared (IR) spectroscopy is a chemical analytical technique, which measures the infrared intensity versus wavelength (wavenumber) of light. Based upon the wavenumber, infrared light can be categorized as far infrared (4 ~ 400cm-1), mid infrared (400 ~ 4,000cm-1) and near infrared (4,000 ~ 14,000cm-1).Infrared spectroscopy detects the vibration characteristics of chemical functional groups in a sample. When an infrared light interacts with the matter, chemical bonds will stretch, contract and bend. As a result, a chemical functional group tends to adsorb infrared radiation in a specific wavenumber range regardless of the structure of the rest of the molecule. Significance in Carbon nanotubes: Although CNTs have active modes in infrared region , fourier transform infrared spectroscopy (FT-IR) is mainly used to characterize absorbed on CNTs ( e.g. NO2, NH3,H2,N2 etc. ) and also to evaluate all the modifications of the CNTs structure and the nature of the compounds added to the CNT surface by fictionalization treatment. Infrared spectroscopy can also give informations about impurities remaining from synthesis. The schematic below represents the set-up of the system
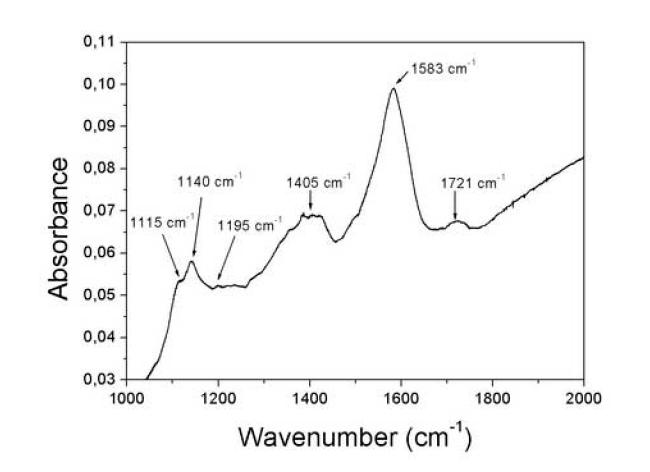
CNT FT-IR Spectra
|