Lab-On-a-Chip
Microfluidics for Lung Cancer Treatment
In this research activity we intend to create an innovative microfluidic-based technological platform aimed at supporting the diagnosis and treatment of lung cancer, using non-small cell lung cancer as a case study (NSCLC), as it is the most frequent type of histology and subjected to routine surgical resection when identified at an early stage.
The ongoing activities concern the development and integration of multiple innovative technologies to support the development of Lab-on-Chip and Organ-on-Chip technology demonstrators and biosensors for the early detection of tumor biomarkers.
A part of the prototypes will have diagnostic purposes, in particular it will be aimed at the early diagnosis of thoraco-pulmonary tumors through different strategies (liquid biopsy, detection of exosomes and tumor biomarkers with advanced high sensitivity biosensors based on electrochemical transduction methods). These strategies are implemented in Lab-on-Chip devices for the analysis of both biological fluids and organoid culture fluids.
A second aim is therapeutic, through the development of Organ-on-Chip prototypes dedicated to in vitro culture in 3D printed structures of organoids deriving from pathological patients, in order to carry out a screening of the currently available active drugs. The ambition is to prove that the organoids retain the characteristics of the primary tumor in the Organ-on-Chip to be used for diagnostic and classification purposes, as well as for future drug screening.



Contact information
Matteo Cocuzza
matteo.cocuzza@infm.polito.it
Simone Marasso
simone.marasso@polito.it
Free Flow Electrophoresis (FFE)
A precision medicine for early diagnosis and detection of diseases needs the development of smart devices able to isolate biomarkers from biological fluids, to achieve a clear definition of their biological function and as well as for diagnostic purposes. Since its introduction in the 1960s Free Flow Electrophoresis approach results to be a separation technique suitable for preparative scale fractionation and separation of a large range of species: solids particles, cells, organelles and comples protein mixtures, etc. In details, FFE is a continuous and analytical separation technique used to separate chemical species, micro and nanoparticles and biomolecules in a flowing stream according to size and charge. A thin sample stream is introduced into a planar separation channel with buffer running in parallel: when the electric field is applied perpendicularly across the separation chamber, charged analytes deflect laterally based on their electrophoretic mobility. In our research activity we are working on developing a Lab-On-a-Chip device based on the FFE approach operating at the microscale level (µFFE), thus acquiring the several weel-known advantages related to the scaling dimensions. Currently we are investigating devices fabricated through standard soft lithography process and by three-dimensional (3D) printing technology and analysing which one owns the best results both concerning fabrication process and performances.

Contact information
Federica Barbaresco
matteo.cocuzza@infm.polito.it
Simone Marasso
simone.marasso@polito.it
3D Printed Microfluidics
Since the introduction of Lab-On-Chip devices in the early 1990s, silicon and glass have been the dominant substrate materials for their fabrication. This was primarily driven by the fact that fabrication methods were well established and surface properties and functionalization methods were well characterized and developed. However, the cost of producing systems in silicon or glass was driving commercial producers to seek other materials like polymers that involve reduced costs and simplified manufacturing procedures (applicable to mass replication technologies). The introduction of polymer technology allows to overcome the disadvantages linked to the rigid silicon processing.
In this perspective, 3D printing promises to be an effective alternative to micromachining as it allows to print not only a single-layer microfluidic device with the desired geometry, but a multi-layered more complex microfluidic chip, eventually embedding external electrical/mechanical components.
We are currently working on this topic, implementing processes to build micro- and nano-fluidic structures with high-resolution additive manufacturing techniques. By Micro StereoLithography (SL) and DLP 3D Printing we fabricate microfluidics and Lab-On-a-Chip with the advantages to easily pass from the design to the device avoiding the implementation of high cost processes or micromachining technology.
The straightforward researches developed in our laboratories are focused both on materials science and technology aspects. Indeed, we aim to further exploit the synergy between freedom of design typical of the 3D printing technologies and the material properties and, in parallel, to move towards the integration of different printing technologies.
From the materials point of view new non-toxic materials are investigated.
Silicon-like photocurable formulations are studied to obtain flexible microfluidic chips with micrometric resolution as well as rigid materials are investigated for the study of smart multiwell plates for pharmacological studies.
Furthermore, the choice of specific formulations’ components enable to 3D print objects presenting reactive surfaces. This allows the development of fast and selective post functionalization processes that can lead to the production of smart microfluidic detection devices.


Concept of the functionalizable 3D printed microfluidic chip Modular 3D printed Lab-On-a-Chip for cancer early detection
Novel printing strategies are on the way to integrate SL and DLP fabrication with two-photon polymerization (2PP) to build features with a spatial resolution down to 120 nm. 2PP is combined with faster techniques so that most of the device is obtained by a lower resolution and more efficient approach (SL), while the 3D micro-/nano- feature is printed by 2PP, thus shortening process times. The adopted novel printing strategy allows for maximizing the printing resolution with respect to printing velocity.

Contact information
Ignazio Roppolo
Tel. +39 011 090 7412
ignazio.roppolo@polito.it
Annalisa Chiappone
Tel. +39 011 090 7412
annalisa.chiappone@polito.it
Valentina Bertana
valentina.bertana@polito.it
Microfluidics for Oil Industry Applications
The characterization of reservoir fluids from the point of view of chemical composition and thermodynamic behavior is one of the key elements for the study, understanding and prediction of the production behavior of a hydrocarbon reservoir.
Samples of liquids from the reservoir come in the form of mixtures of hydrocarbons, often emulsified with water. However, the correct determination of the composition of the hydrocarbon mixture requires that it be pure, i.e. the aqueous phase is appropriately separated before chemical analysis such as infrared spectroscopy.
The analysis of the chemical composition of the reservoir fluids is carried out at different times both in specialized laboratories, and therefore in ideal working conditions, but also on-site and near the production plant. It is therefore particularly interesting to develop a small, and therefore transportable, instrument capable of performing the separation and characterization of the product oil reliably, in a short time and by using small volumes of fluid samples.
The aim of the research is to verify the feasibility, design, implementation and validation of a solution, based on microfluidic devices, aimed at the capillary separation of the oil and water phases for the subsequent characterization of the reservoir hydrocarbon mixture by infrared spectroscopy.
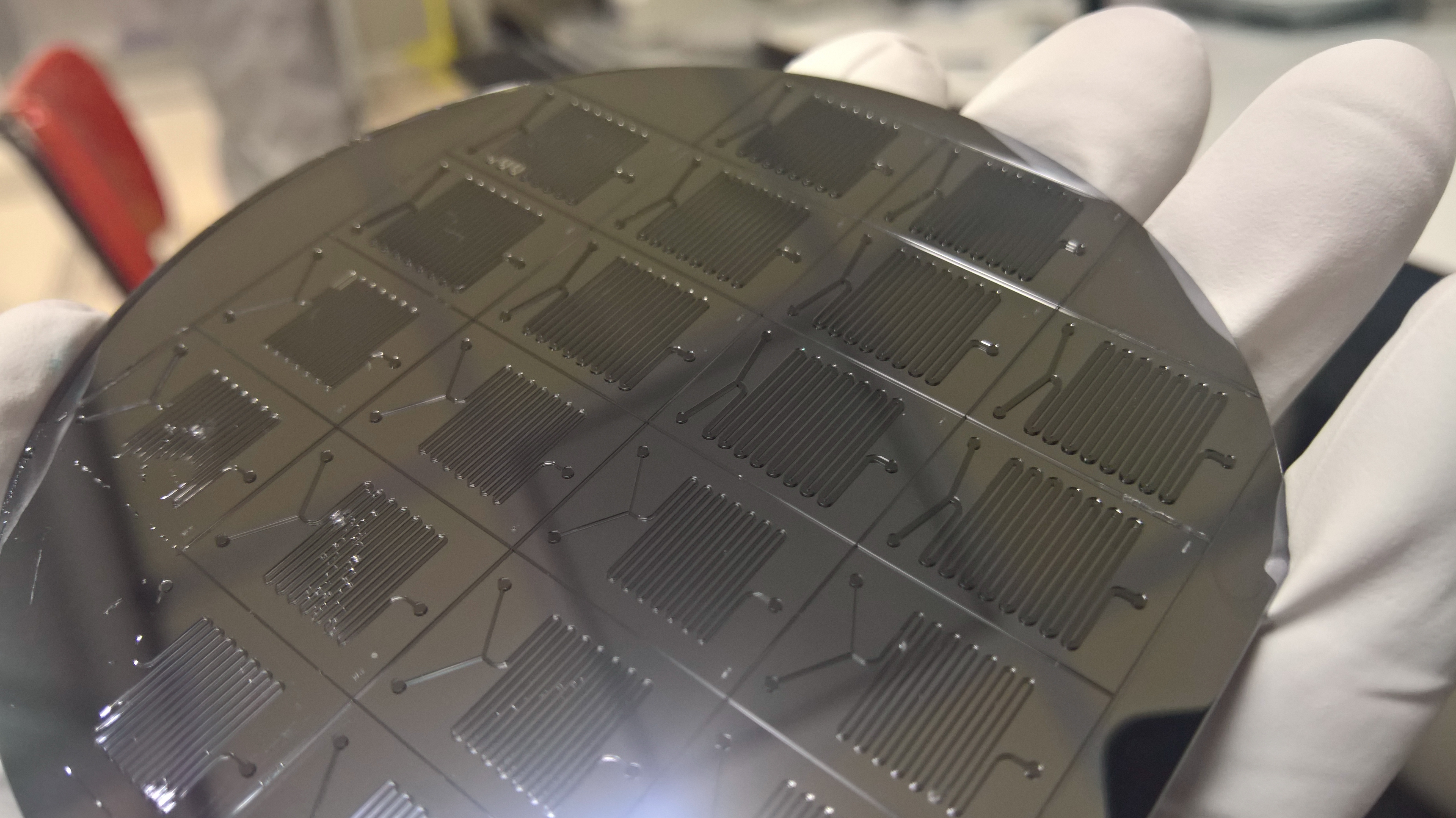

Contact information
Matteo Cocuzza
matteo.cocuzza@infm.polito.it
Simone Marasso
Publications
-
"A modular 3D printed lab-on-a-chip for early cancer detection", A Chiadò, G Palmara, A Chiappone, C Tanzanu, CF Pirri, I Roppolo, F Frascella, (2020) Lab Chip, 20, 665-674 Doi: 10.1039/C9LC01108K
-
"Real-time and reversible light-actuated microfluidic channel squeezing in dye-doped PDMS", A Angelini, U Agero, F Ferrarese Lupi, M Fretto, F Pirri, F Frascella, Soft Matter, 2020, doi.org/10.1039/D0SM00217H
-
"Simple PDMS microdevice for biomedical applications", C. Potrich, L. Lunelli, M. Cocuzza, S.L. Marasso, C.F. Pirri, C. Pederzolli, Talanta, Vol. 193, 2019, pp. 44-50, doi:10.1016/j.talanta.2018.09.080
-
"Photofabrication of polymeric biomicrofluidics: New insights into material selection", E. Fantino, A. Chiadò, M. Quaglio, V. Vaghi, M. Cocuzza, S.L. Marasso, C. Potrich, L. Lunelli, C. Pederzolli, C.F. Pirri, R. Bongiovanni, A. Vitale, Materials Science and Engineering: C, 2019, 110166, https://doi.org/10.1016/j.msec.2019.110166
-
"A passive two-way microfluidic device for low volume blood-plasma separation", Spigarelli, L., Bertana, V., Marchisio, D., Scaltrito, L., Ferrero, S., Cocuzza, M., Marasso, S.L., Canavese, G., Pirri, C.F., Microelectronic Engineering, 2019, 209, pp. 28-34, DOI: 10.1016/j.mee.2019.02.011
-
"Optimization of a suspended Two Photon Polymerized microfluidic filtration system", F. Perrucci, V. Bertana, S.L. Marasso, G. Scordo, S. Ferrero, C.F. Pirri, M. Cocuzza, A. El-Tamer, U. Hinze, B.N. Chichkov, G. Canavese, L. Scaltrito, Microelectronic Engineering, 2018, 195, pp. 95-100, DOI: 10.1016/j.mee.2018.04.001
-
"3D-printed microfluidics on thin Poly(methyl methacrylate) substrates for genetic applications", V. Bertana, C. Potrich, G. Scordo, L. Scaltrito, S. Ferrero, A. Lamberti, F. Perrucci, C.F. Pirri, C. Pederzolli, M. Cocuzza, S.L. Marasso, Journal of Vacuum Science and Technology B, 36(1), Jan/Feb 2018, 01A106-1/7, DOI: 10.1116/1.5003203
-
"miRNA purification with an optimized PDMS microdevice: toward the direct purification of low abundant circulating biomarkers", G. C Santini, C. Potrich, L. Lunelli, L. Vanzetti, S. L. Marasso, M. Cocuzza, C. F. Pirri, C. Pederzolli, Biophysical Chemistry, 2017, 229, pp. 142-150, doi: 10.1016/j.bpc.2017.04.009
-
"Optimized design and fabrication of a microfluidic platform to study single cells and multicellular aggregates in 3D", S.L. Marasso, A. Puliafito, D. Mombello, S. Benetto, L. Primo, F. Bussolino, C.F. Pirri, M. Cocuzza, Microfluidics and Nanofluidics, February 2017, 21:29, DOI: 10.1007/s10404-017-1872-0
-
"Oxygen Inhibition Lithography for the Fabrication of Multi-Polymeric Structures", A. Vitale, M. Quaglio, A. Chiodoni, K. Bejtka, M. Cocuzza, C.F. Pirri and R. Bongiovanni, Advanced Materials, 2015, Vol.27(31), pp. 4560-4565, doi:10.1002/adma.201501737
-
"On-chip purification and detection of hepatitis C virus RNA from human plasma", V. Vaghi, C. Potrich, L. Pasquardini, L. Lunelli, L. Vanzetti, E. Ebranati, A. Lai, G. Zehender, D. Mombello, M. Cocuzza, C.F. Pirri, C. Pederzolli, Biophys. Chem., Jan. 2016, 208, pp.54-61, http://dx.doi.org/10.1016/j.bpc.2015.06.005
-
"Blue and UV combined photolithographic polymerization for the patterning of thick structures", E. Fantino, A. Vitale, M. Quaglio, M. Cocuzza, C.F. Pirri, R. Bongiovanni, Chemical Engineering Journal, 2015, Vol. 267, pp. 65-72, DOI: 10.1016/j.cej.2014.12.088
-
Metal-elastomer nanostructures for tunable SERS and easy microfluidic integration", A. Lamberti, A. Virga, A. Angelini, A. Ricci, E. Descrovi, M. Cocuzza and F. Giorgis, RSC Adv., 5, 2015, pp. 4404-4410, DOI: 10.1039/C4RA12168F
-
"PDMS membranes with tunable gas permeability for microfluidic applications", A. Lamberti, S. Marasso, M. Cocuzza, RSC Adv., 4 (106), 2014, pp. 61415-61419, DOI: 10.1039/c4ra12934b
-
"OncomiR detection in circulating body fluids: a PDMS microdevice perspective", C. Potrich, V. Vaghi, L. Lunelli, L. Pasquardini, G.C.Santini, C. Ottone, M. Quaglio, M. Cocuzza, C.F. Pirri, M. Ferracin, M. Negrini, P. Tiberio, V. De Sanctis, R. Bertorelli and C. Pederzolli, Lab Chip, 14 (20), 2014, pp. 4067-4075, DOI: 10.1039/C4LC00630E
-
"A polymer Lab-on-a-Chip for genetic analysis using the arrayed primer extension on microarray chips", S.L. Marasso, D. Mombello, M. Cocuzza, D. Casalena, I. Ferrante, A. Nesca, P. Poiklik, K. Rekker, A. Aaspollu, S. Ferrero, C.F. Pirri, Biomed. Microdev., Vol. 16(5), 2014, pp.661-670, DOI: 10.1007/s10544-014-9869-x
-
"Direct Photolithography of Perfluoropolyethers for Solvent-Resistant Microfluidics", A. Vitale, M. Quaglio, S. L. Marasso, A. Chiodoni, M.Cocuzza and R. M. Bongiovanni, Langmuir, 2013, 29 (50), pp 15711–15718, DOI: 10.1021/la402755q
-
"Siloxane photopolymer to replace polydimethylsiloxane in microfluidic devices for Polymerase Chain Reaction", A.Vitale, M. Quaglio, S. Turri, M. Cocuzza, R. Bongiovanni, Polym. Adv. Technol., 24, 2013, pp. 1068–1074, DOI: 10.1002/pat.3189
-
"Liposomes sensing and monitoring by Organic Electrochemical Transistors integrated in microfluidics", G. Tarabella, A.G. Balducci, N. Coppedè, S. Marasso, S. Barbieri, M. Cocuzza, P. Colombo, R. Mosca, F. Sonvico, S. Iannotta, Biochimica et Biophysica Acta (BBA) - General Subjects, 1830, 2013, pp. 4374-4380, 10.1016/j.bbagen.2012.12.018
-
"Photopolymerization of a perfluoropolyether oligomer and photolithographic processes for the fabrication of microfluidic devices", A. Vitale, M. Quaglio, M. Cocuzza, C.F. Pirri, R. Bongiovanni, Eur Polym J, 2012, 48 (6), pp. 1118 - 1126, doi:10.1016/j.eurpolymj.2012.03.016
-
"Solid phase DNA extraction on PDMS and direct amplification", L. Pasquardini, C. Potrich, M. Quaglio, A. Lamberti, S. Guastella, L. Lunelli, M. Cocuzza, L. Vanzetti, C. F. Pirri, C. Pederzolli, Lab Chip, 2011, 11 (23), 4029 - 4035, DOI: 10.1039/c1lc20371a
-
“Cost efficient master fabrication process on copper substrates”, S. Marasso, G. Canavese, M. Cocuzza, Microelectronic Engineering, Vol. 88(8), August 2011, Pages 2322-2324, doi:10.1016/j.mee.2011.02.023
-
"Elastomeric nanocomposite based on Carbon Nanotubes for Polymerase Chain Reaction device", M. Quaglio, S. Bianco, R. Castagna, M. Cocuzza, C.F. Pirri, Microelectronic Engineering, Vol. 88(8), August 2011, Pages 1860-1863, doi:10.1016/j.mee.2011.01.032
-
"A Multilevel Lab On Chip platform for DNA analysis", S. L. Marasso, E. Giuri, G. Canavese, R. Castagna, M. Quaglio, I. Ferrante, D. Perrone, M. Cocuzza, Biomedical Microdevices, Volume 13, Issue 1 (2011), Page 19 (doi: 10.1007/s10544-010-9467-5)
-
"Evaluation of different PDMS interconnection solutions for silicon, pyrex and COC microfluidic chips", G. Canavese, E. Giuri, S.L. Marasso, D. Perrone, M. Quaglio, M. Cocuzza, C.F. Pirri, J. Micromech. Microeng., 18 (2008) 055012
-
"APEX protocol implementation on a Lab-on-a-chip for SNPs detection", S. Marasso, G. Canavese, S. Lobartolo, M. Cocuzza, A. Ferrarini, E. Giuri, D. Perrone, M. Quaglio, A. Ricci, I. Vallini, Microelectronics Engineering, 85 (2008), 1326-1329 (doi:10.1016/j.mee.2007.12.024)



